Electron Probe MicroAnalysis (EPMA) uses Wavelength-Dispersive X-ray Spectroscopy (WDS) and Energy-Dispersive X-ray Spectroscopy (EDS) to identify the elements in a sample. WDS is based on diffraction from crystals to isolate wavelengths of characteristic X-rays, which are then measured by gas-flow and sealed proportional counters. EDS is based on measuring X-rays of all wavelengths using a semi-conductor detector. Typically, WDS is used for quantitative analysis, whereas EDS is used for rapid identification of elements. In addition, EDS has less resolving power to discriminate between elements with peaks at similar energy levels, as well as lesser peak-to-background ratios.
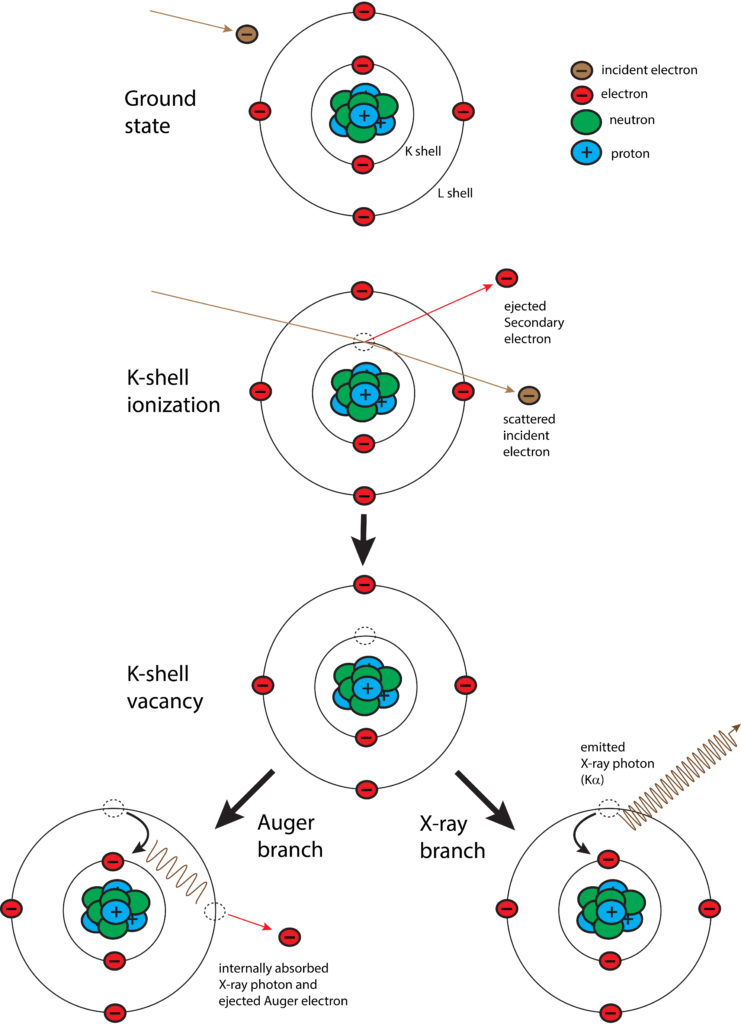
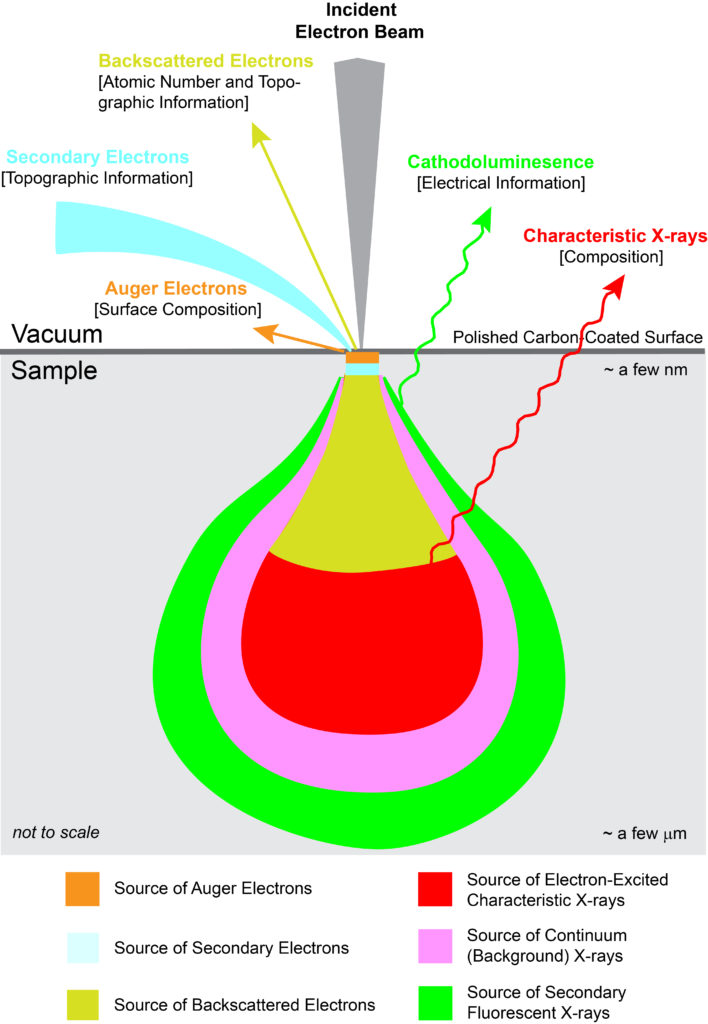
With EPMA, a sample is placed under vacuum and then bombarded with a focused beam of electrons. These electrons interact repeatedly with the atoms in the sample until they either come to rest within or exit from the sample. If an incident electron ionizes an atom by ejecting an inner orbital electron via inelastic scattering, an X-ray photon is produced as the atom de-excites by cascading the vacancy to an outer electron shell. The energy of the X-ray photon is unique, or characteristic, to the given element at the sampling location. By convention, characteristic X-rays are named by the ionized shell (e.g., K, L, M) and the rank energy of the outer shell that provides the replacement electron (highest energy–?, ?, ?, etc.–lowest energy).
In addition to generation of characteristic X-rays, other interactions within the sample occur. Most notably are BackScattered Electrons (BSE) and Secondary Electrons (SE). A backscattered electron is an incident electron that is elastically scattered by the nucleus such that it remerges from the sample with high energy. Backscattering is strongly dependent on the average atomic number at the sampling location. By measuring backscattered electrons over a sample, a compositional map (BSE image) is produced where heavier atoms show brighter and lighter ones darker. A secondary electron is a loosely bound electron near the surface of a sample. This electron gains sufficient energy via inelastic scattering from the incident beam to escape the sample, albeit at low energy. By measuring secondary electrons over a sample, a surface topographic map (SE image) is produced.
Generation of Characteristic X-rays by Electron Transition
Schematic diagram of the process for generation of characteristic X-rays. An incident (beam) electron ejects an orbital electron (K shell) via inelastic scattering, which leaves the atom in an excited state of energy. The energy is lowered by either Auger process or X-ray photon emission. In the former case, X-ray photon energy is absorbed by another orbital electron (L shell in above scenario), which is then ejected. In the latter case, a characteristic X-ray is emitted.
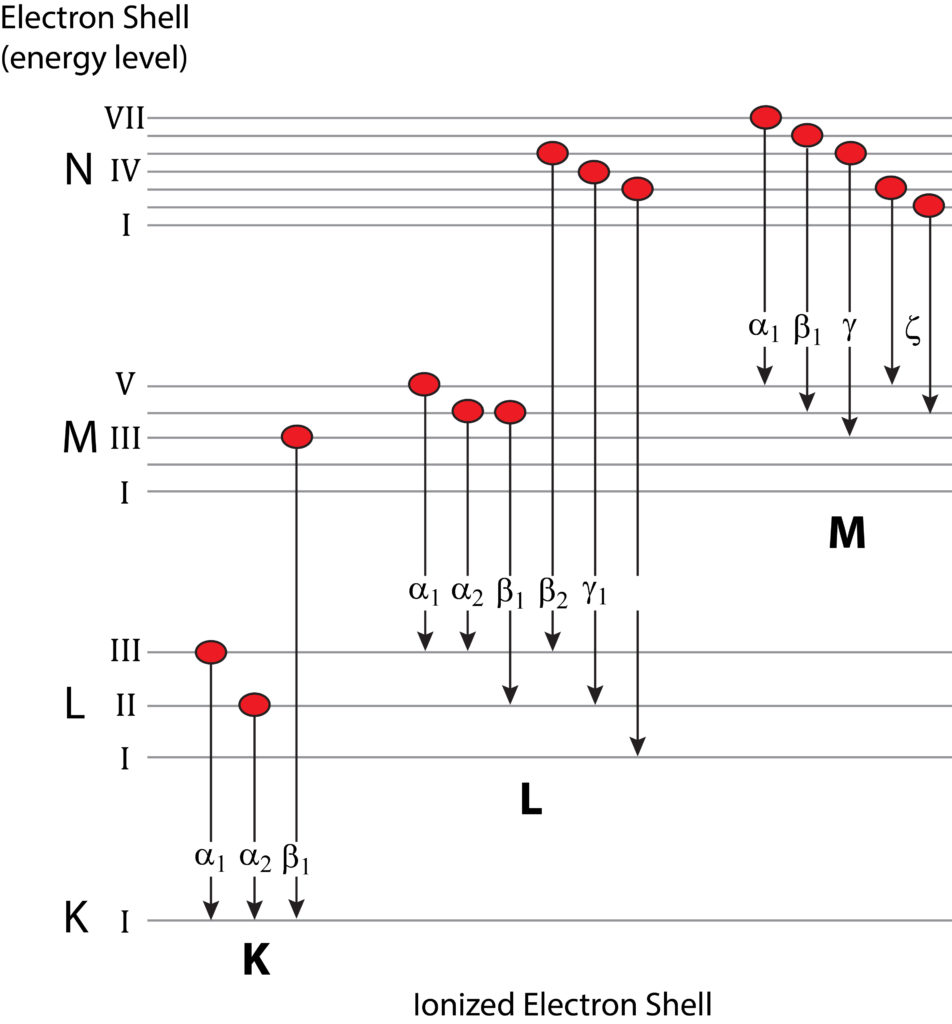
Nomenclature for Characteristic X-rays
Generation of characteristic X-rays through electron transition whereby an outer-shell electron occupies the vacancy created by the ejection of a secondary electron from a more inner ionized shell. The X-ray is labelled according to the ionized shell and the rank energy of the shell providing the replacement electron (e.g., K?1, K?2, K?1, etc.).
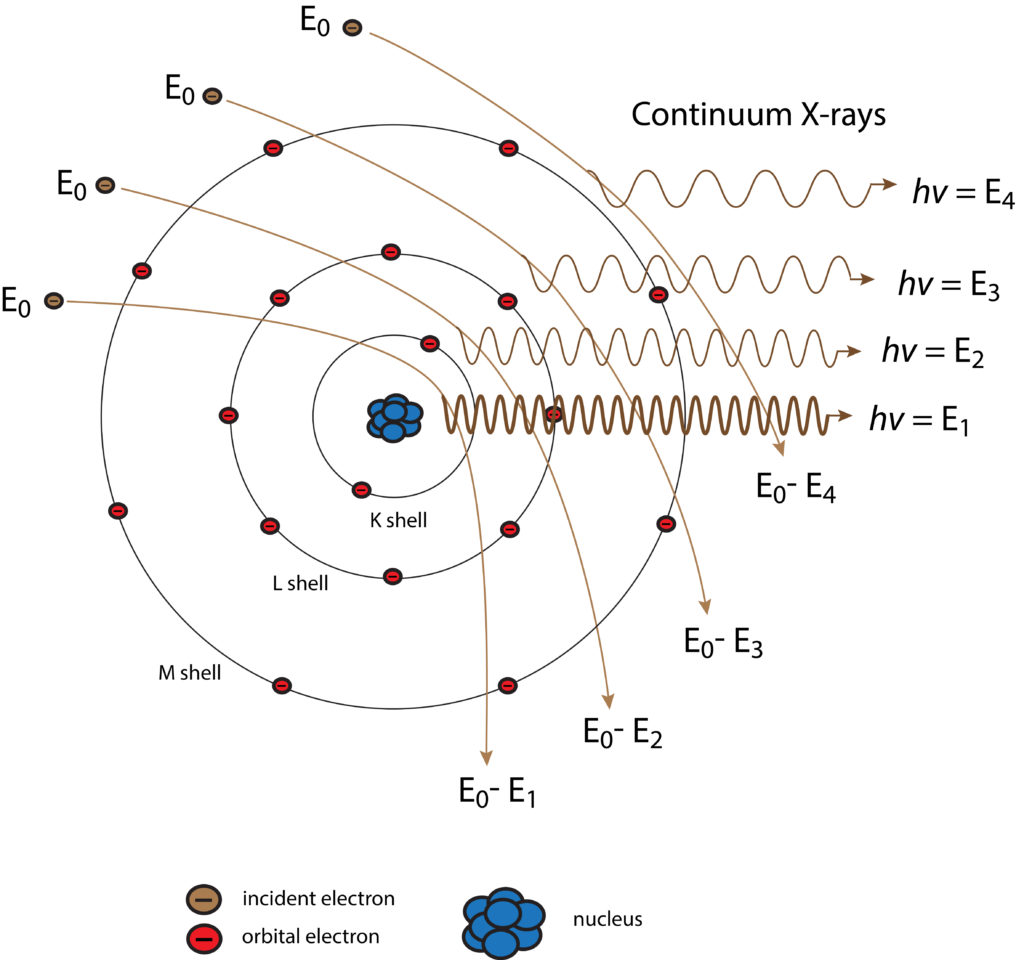
Generation of Continuum (Background) X-rays
Schematic diagram of the generation of continuum X-rays. The background level of radiation determines the overall detection level.
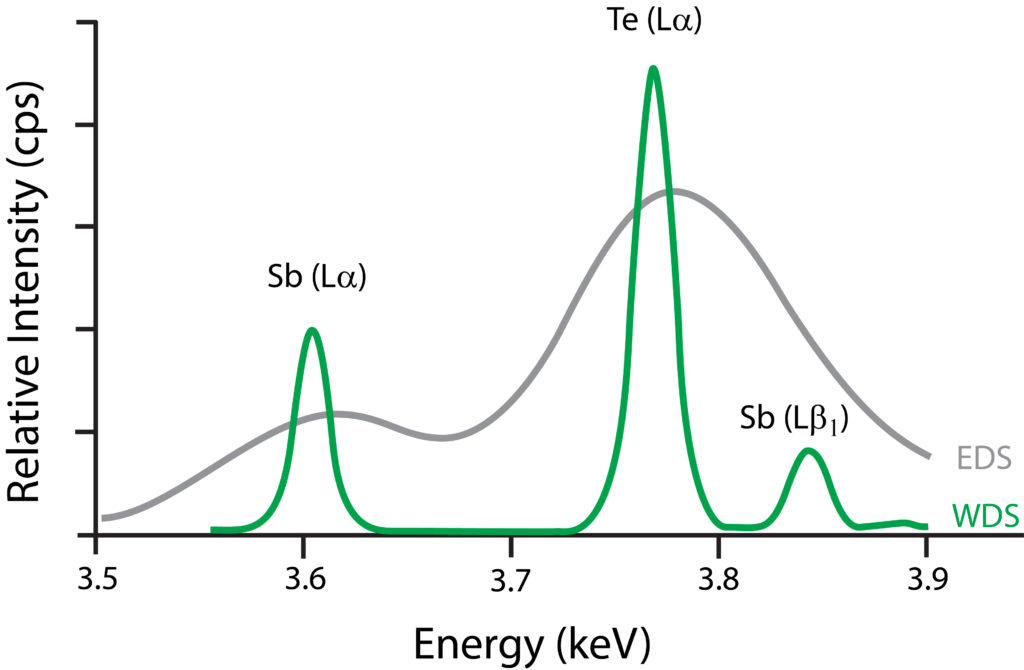
Resolving Capabilities: WDS Versus EDS
Graph showing resolving capabilities between WDS and EDS. In this example, the former is able to discriminate between Tellurium’s L? peak and Antimony’s L?1 peak. In addition, the latter has lesser peak-to-background levels.